Europain Survey Protocol
Important messages for recruitment of patients
Reminder for inclusion period
- Verify period of recruitment with your National Principal Investigator and Principal Investigators
- Inclusions start at 0:00 of the first day of the inclusion period and end at 23:59 the last day of this period
- Monitor admission in the unit using the UNIT LOG. This allows to register all admitted neonates and those included in the study.
- For all NEWLY admitted patients, enter required data on the web based questionnaire. The server will generate an identification number.
- In case of difficulty entering data directly from the patient's files, a PAPER COPY of the online questionnaire can be used to gather data and enter it subsequently on the web based questionnaire.
- In case of doubt or problem, please email emilie.courtois@trs.aphp.fr
- The inclusion period will be determined FOR EACH unit in agreement with the National Principal Investigator.
- Some Units, in some countries, will start inclusions on October 1st, 2012; others will do that later.
- During the study period (ONE MONTH- 31 days) ALL NEWLY ADMITTED BABIES in the unit are included, the differences between ventilated and non ventilated babies will be done during the data analysis.
- The duration of data collection for every included infant is 28 days. However, data collection will stop before 28 days if the infant leaves the unit (discharge, death, transfer to another hospital).
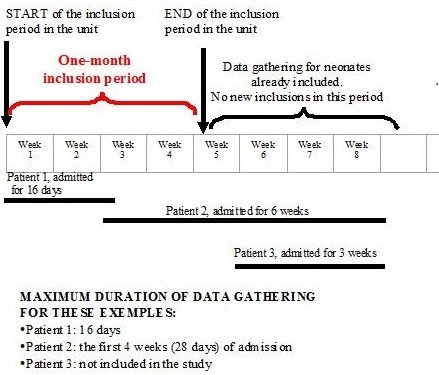
Diagram of inclusion and data gathering period
The diagram below shows the inclusion period and the length of data collection
Timeline illustrating an example of data collection periods for three infants (black bars). No infant is included after the one-month inclusion period but can, once included, generate data for a maximum of four weeks
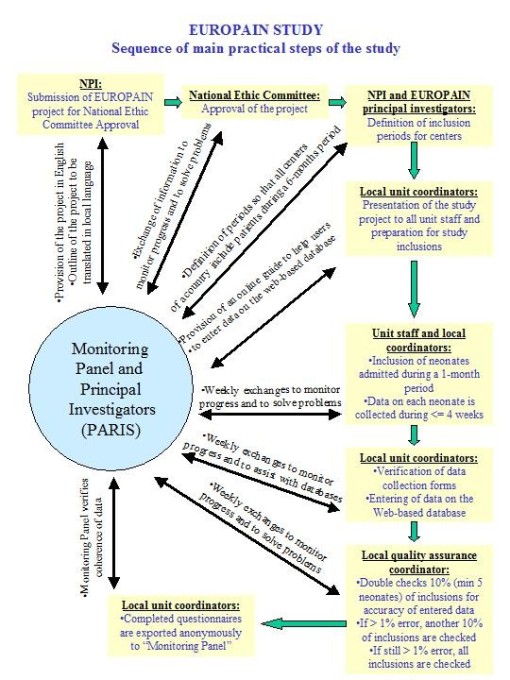
Diagram of main organisational steps
Data quality checking
Quality control will be performed as a self-audit at the local hospitals.
This quality control will be carried out by the quality control coordinator (independent person designated by the medical coordinator). It will be conducted on 10% of included neonates with a minimum
of 5 neonates. A list of patients whose files are to double check will be sent to medical coordinator. The patients to verify will be chosen randomly by the EUROPAIN STUDY principal investigators. We
set up a limit of 1% errors in demographic and medical data. If this limit is exceeded, then another 10 % of charts should be checked. If still more than 1 % mistakes are found, all the charts should
be double-checked. Found mistakes should, of course, be corrected on the data base.
Outlines of the EUROPAIN SURVEY PROTOCOL
An outline of the EUROPAIN SURVEY is available in different languages:
EUROPAIN protocol V5 on Feb 28_2012 OUTL[...]
Document Adobe Acrobat [43.0 KB]
Résumé en français EUROPAIN SURVEY.pdf
Document Adobe Acrobat [157.6 KB]
EUROPAIN_STUDY vers German.pdf
Document Adobe Acrobat [30.0 KB]
EUROPAIN protocol V5 on Feb 28_2012 ITAL[...]
Document Adobe Acrobat [43.3 KB]
EUROPAIN_protocol_V5_on_Feb_28_2012_Swed[...]
Document Adobe Acrobat [56.1 KB]
EUROPAIN protocol Finnish version.pdf
Document Adobe Acrobat [64.8 KB]
EUROPAIN protocol V5 SPANISH on Feb 28_2[...]
Document Adobe Acrobat [247.7 KB]
EUROPAIN protocol V5 on Feb 28_2012_Norw[...]
Document Adobe Acrobat [60.2 KB]
Europain Protocol Greek Version.pdf
Document Adobe Acrobat [102.4 KB]
EUROPAIN protocol with Dutch Summary.pdf
Document Adobe Acrobat [194.4 KB]
Europain protocol Lithuanian version.pdf
Document Adobe Acrobat [179.5 KB]
EUROPAIN Protocol Polish Version.pdf
Document Adobe Acrobat [72.4 KB]
Europain protocol Portuguese version.pdf
Document Adobe Acrobat [217.6 KB]